find the electron configuration ge|Electron Configuration Calculator : Pilipinas Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit pa 当サイトからKKPokerに登録するとポーカーラボの限定クラブに加入でき、様々な限定特典が貰えます。 また、当クラブでは 年間500万円以上のプレゼントイベントが定期的に開催 されています。 【当サイト限定特典】 ・ハンド数に応じてMAX50%のレーキバック ・ポーカーラボ限定フリーロールへ .
PH0 · How to Write the Electron Configuration for Germanium (Ge)
PH1 · Germanium Electron Configuration (Ge) with Orbital
PH2 · Germanium (Ge) [32] — Chemical Element — Periodic Table
PH3 · Germanium
PH4 · Find the Electron Configuration Ge
PH5 · Electron Configuration for Germanium (Ge, Ge2+, Ge4+ ions)
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration Calculator
PH8 · Electron Configuration Calculator
PH9 · 2.4 Electron Configurations
Yati Barangay Hall in Liloan There are 85,071 City Services in the database. To help find what you are looking for, there is a handy search helper.
find the electron configuration ge*******The electron configuration shows that the last shell of germanium has four electrons. Therefore, the valence electrons of germaniumare four. There are two types of germanium ions. The germanium atom exhibits Ge2+ and Ge4+ ions. The germanium atom donates two electrons in the 4p . Tingnan ang higit pa
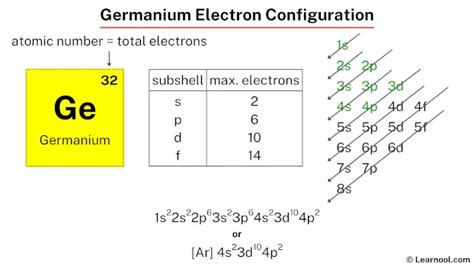
The total number of electrons in germanium is thirty-two. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in germanium in specific rules in different . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy . Tingnan ang higit paGe G e. The electron configuration is the arrangement of electrons of an atom. It concerns the way electrons are distributed in the orbitals of the atom. There are 4 4 .
177. 29K views 3 years ago. A step-by-step description of how to write the electron configuration for Germanium (Ge). In order to write the Ge electron configuration . Mar 23, 2023
FAQ. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine .Find the Electron configuration of any Element on the Periodic Table of Elements with this simple, yet very useful widget. Send feedback | Visit Wolfram|Alpha. Get the free .
Get the facts about element Germanium (Ge) [32] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data .
Germanium Electron Configuration (Ge) with Orbital Diagram. Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of .2020-11-13 by Nick Connor. Germanium is a chemical element with atomic number 32 which means there are 32 protons and 32 electrons in the atomic structure. The .1. Find the electron configuration of the following: a) silicon: [Ne] 3s 2 3p 2. b) tin: [Kr] 5s 2 4d 10 5p 2. c) lead: [Xe] 6s 2 4f 14 5d 10 6p 2. 2. Scenario: You are currently studying the element iodine and wish to use .

The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .Electron Configuration Calculator The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .Germanium. 32. 72.630. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.
Where, 4p 2 indicates that the 4p subshell has 2 electrons. Therefore, the final electron configuration of germanium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 2. And the condensed/abbreviated electron configuration of germanium is [Ar] 4s 2 3d 10 4p 2. Where, Ar is argon.
The atomic number of germanium is 32, which means it has 32 electrons. Now it is possible to find the orbital notation of germanium very easily through electron configuration. That is, the orbital notation of germanium is 1s .
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait . The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups .find the electron configuration ge Electron Configuration Calculator Germanium is a chemical element of the periodic table with chemical symbol Ge and atomic number 32 with an atomic weight of 72.6308 u and is classed as a metalloid. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 2: Electrons per shell: 2, 8, 18, 4: Valence electrons : 4: Valency electrons : 4,2:
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the .
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 2.6.6 2.6. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series: Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32. It is a hard, lustrous, greyish-white metalloid of the . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add .Its electronic configuration is 1s2, 2s2, 2p6, 3s1 The largest value of the Principle Quantum Number (n) is 3, so that is the outermost orbital. Counting the number of electrons, we find that only the s orbital is present and it has only one electron. So Na has one electron in its outermost orbital. Another example that I'll use is Fluorine (F). 2. Find your atom in the ADOMAH table. To write electron configuration of an element, locate its symbol in ADOMAH Periodic Table and cross out all elements that have higher atomic numbers. For example, if you need to write electron configuration of Erbium (68), cross out elements 69 through 120.Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
Electron configurations help you to do this. To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the . Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.
2022-11-09 05:04:04 Kimora : Come let's fuck . 2022-04-09 22:15:05 Derek: Wish I was there to lick your pussy after fucking your dog. 2022-03-09 15:11:22 Lina:
find the electron configuration ge|Electron Configuration Calculator